For patients living in Japan
Hernicore®
Injection therapy for lumbar disc herniation
This treatment uses an enzyme-containing drug (Hernicore®) injected directly into the intervertebral disc to shrink the nucleus pulposus of the disc, which is the source of the herniation.
About Hernicore®
Differences from endoscopic spine surgery
Cases in which the treatment is expected to be effective
What is Hernicore®?
This is a treatment in which an enzyme(Condoliase)-containing drug (Hernicore®) is injected directly into the intervertebral disc to shrink the nucleus pulposus of the disc, the source of the herniation, thereby reducing the pressure on the nerves. At present, this therapy has been approved only in Japan. The nucleus pulposus is rich in components that retain water. When injected, condoliase, the active ingredient in Hernicore®, breaks down these components in the nucleus pulposus and relieves the swelling caused by water. As a result, pressure on the nerves is reduced, and symptoms such as pain and numbness can be alleviated.
Differences from endoscopic spine surgery
The standard method of treating hernias in our hospital is endoscopic procedures, such as MED (Microendoscopic Discectomy) and FED (Full Endoscopic Discectomy). In endoscopic surgery, the protruding hernia is directly removed while viewing the endoscopic image. In principle, our group performs endoscopic procedures under general anesthesia. Although it varies from person to person, a surgical wound is made, and the patient will experience pain in the wound area for a while. In addition, hospitalization for at least three days is required. Hernicore®, on the other hand, is performed under local anesthesia, and the wound is about the size of a needle hole and is hardly noticeable. It is a one-day procedure in principle, and the patient can return home on the day of treatment.
However, Hernicore® is not suitable for people with allergies, as it can induce an anaphylactic reaction. Furthermore, the disadvantages are that the types of hernia indicated for this treatment are limited, and that because the part of the hernia that is compressing the nerve is not directly removed, the efficacy rate is lower than that of endoscopic spine surgery.
However, Hernicore® is not suitable for people with allergies, as it can induce an anaphylactic reaction. Furthermore, the disadvantages are that the types of hernia indicated for this treatment are limited, and that because the part of the hernia that is compressing the nerve is not directly removed, the efficacy rate is lower than that of endoscopic spine surgery.
| Anesthesia | Length of hospital stay |
Wound | Efficacy rate | Other | |
|---|---|---|---|---|---|
| Hernicore® | Local anesthesia | 0.5-1 day | Needle hole | 60-70% |
|
| Endoscopic spine surgery |
General anesthesia | 3-6 days | 8-20 mm | 90% or more | Postoperative follow-up and consultation are required |
Cases in which the treatment is expected to be effective
In our group, the results of treatment in young patients are excellent. It can also be effective in treating cases with T2 hyperintensity in the prominent nucleus pulposus and relatively large hernias with spinal canal occupancy of 40% or more.
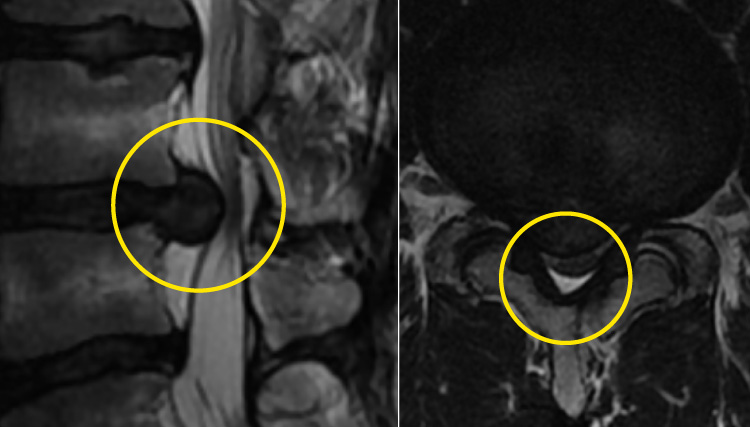
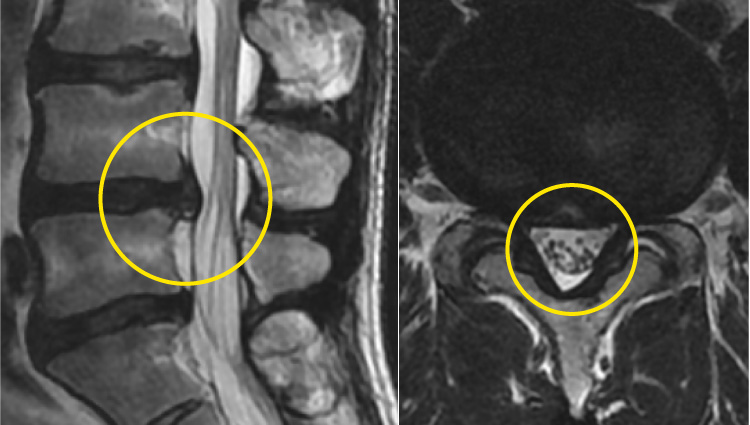
Changes in MRI images before and after the procedure
These MRI images show the changes before and after treatment in a 37-year-old male. Before the procedure, a large hernia was compressing the nerve, but after being treated, the hernia had shrunk and the pressure on the nerve had been reduced.
Pretreatment
Posttreatment
Procedures for Hernicore® injection therapy
Patient is transferred to the operating room
Patient lies down on the operating table
Determination of the site of insertion of the needle
Disinfection and local anesthesia
Needle is inserted into the disc and Hernicore® is injected
Patient rests in the operating room for 30 minutes, then returns to the hospital room
Results of Hernicore® Injection Therapy
Results from our group
Our group has administered Hernicore® therapy to more than 100 patients from August 2018. There have been no adverse events to date. We participated in the clinical trials conducted by the distributors, Seikagaku Corporation and Kaken Pharmaceutical, which demonstrated certain efficacy and safety.
Articles by our group
We have published papers on the efficacy of Hernicore® and the results of our group's treatment.
- Chemonucleolysis with Chondroitin Sulfate ABC Endolyase for Treating Lumbar Disc Herniation: Exploration of Prognostic Factors for Good or Poor Clinical Outcomes
- Chemonucleolysis with chondroitin sulfate ABC endolyase as a novel minimally invasive treatment for patients with lumbar intervertebral disc herniation
Please click here to view the Hernicore® Drug Information Sheet from the distributing pharmaceutical companies.
Precautions for Administration of Hernicore®
- The administration of Hernicore® may result in the occurrence of anaphylaxis (skin symptoms such as itching and urticaria, gastrointestinal symptoms including abdominal pain and nausea, and visual symptoms such as loss of visual field). People with allergies should exercise caution regarding treatment with Hernicore®.
- Patients who have previously received intradiscal enzyme injection therapy (Hernicore® injection) may not undergo this procedure again.
- This is for hernias that are resistant to conservative treatment. Depending on the shape and location of the hernia, intradiscal enzyme injection therapy (Hernicore® injection) may not be indicated.
| Not indicated | Not very effective *Based on the results of our hospital |
Risk of worsening |
|---|---|---|
|
|
|
